Lung cancer frequently metastasizes to other parts of the body. One of the most dangerous areas it can travel to is the brain, significantly reducing life expectancy. Unfortunately, these metastases are common. Up to 7% of people already have cancer cells in the brain when they are first diagnosed with non-small cell lung cancer (NSCLC), and 20% to 40% of those with NSCLC will develop this complication during disease progression. Brain metastases occur in stage 4 lung cancer. Stage 4 lung cancer has a poor prognosis, with life expectancy usually being under a year1.
Currently there are no targeted therapies specific for brain metastases, and the blood-brain barrier can pose a physiologic impediment to many cytotoxic drugs and antibody-based therapies. Surgical tumor resection and/or radiotherapy are the most used forms of treatment, but the overall increase in survival is small (~4-6 months) and the quality of life following the treatment can be very poor2. Clearly, more effective treatments are needed for this devastating disease. The literature reveals very few preclinical oncology models that facilitate the assessment of metastatic brain disease in the presence of a primary tumor. In recognition of this need, Preclinical Oncology (PCO) has developed two xenograft models to not only address metastatic brain disease via a direct intra-cranial implant, but also couples this with a subcutaneous “primary” tumor to effectively allow the evaluation of the response to treatment at both locations.
Furthermore, being able to assess the pharmacokinetic and/or pharmacodynamics (PK/PD) of targeted therapies in both implant locations at the same time is important. Treatment with targeted therapies could have significantly different penetration and absorption rates which will alter PK/PD assessment across either tissue. Given the unique environment in the brain and the obstacle that the blood brain barrier presents comparison across the two location can be a powerful tool to assess the impact of treatment on established metastatic disease and primary tumor.
PCO has optimized the dual disease induction parameter for two human non-small cell lung carcinoma cell lines; NCI-H1975-luc and PC-9-luc. Both cell lines have been transfected with luciferase to allow for bioluminescence imaging (BLI) to monitor the disease progression for the cells implanted intra-cranially. The subcutaneous “primary” tumors are shielded to prevent overlapping signals. In all studies that involved animals, protocols were conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.
NCI-H1975-Luc
NCI-H1975-Luc was established from a female non-smoker. This cell line is of interest to the research community because of its EGFR-L858R/T790M mutational status. The T790M acquired mutation is found in 50-60% of NSCLC patients that become resistant to approved EGFR inhibitors. Therefore, this model is suitable for evaluating next generation EGFR inhibitors, such as compounds that bind to EGFR regardless of the mutational changes that have occurred, or compounds that bind irreversibly. The EGFR expression profile of NCI-H1975-luc has made it a model of interest for the dual implant method because it allows for the assessment of blood brain barrier penetration of an EGFR targeted small molecule. This evaluation can be done multiple ways; traditional efficacy based on BLI monitoring of the metastatic brain disease coupled with caliper measurements of the subcutaneous tumors and/or pharmacodynamic and pharmacokinetic analysis of the metastatic lesion compared to the primary tumors to assess if drug concentration is different. In either scenario the metastatic brain disease response can then be compared to the “primary” subcutaneous tumor response.
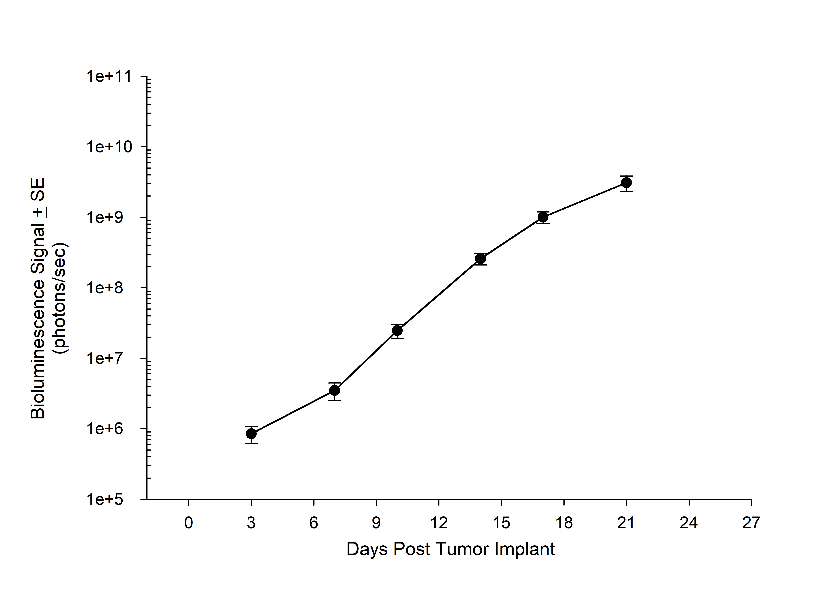
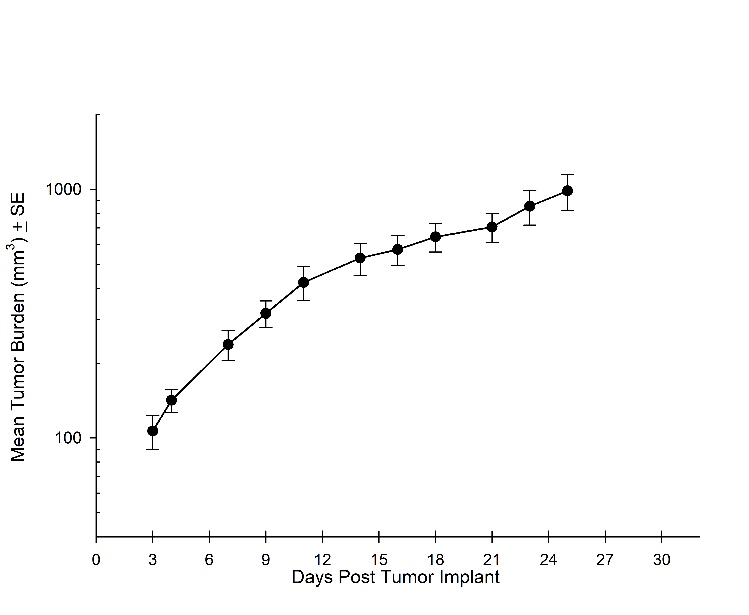
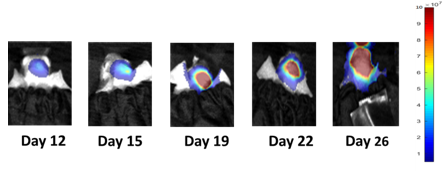
Consistent with the historical tumor growth for this model, the subcutaneous tumor volume doubling time is about 7 days and typically reaching evaluation size (~900mm3) in approximately 25 days post implant (Figure 2). As expected, the model is just as reliable when implanted intra-cranially, with a BLI calculated tumor volume doubling time of 1.6 days (Figure 1 and Image 1).