Posters
Modeling human acute lymphoblastic leukemia (ALL) – using the NALM6-Luc-mCh-Puro model for the development of CAR T cell therapies
01 Jul 2019
Use of CAR T Cell Therapy for B-cell ALL
One such treatment option for B-cell ALL is chimeric antigen receptor (CAR) T cell immunotherapy directed against the CD19 protein. The first FDA-approved CAR T cell therapy was Kymriah (tisagenlecleucel), which was approved on August 30, 2017 for the treatment of pediatric or young adult (up to 25 years of age) relapsed or refractory B-cell ALL. This novel therapeutic approach currently results in an overall response rate of 81% at three months and a durable remission rate of 59% at 12 months, thereby providing a potentially curative option for patients who otherwise had relapsed after, or are refractory to, standard treatment regimens.
While these response rates in a relapsed or refractory patient population are very impressive, accumulating experience demonstrates that remissions may not be complete in a significant number of patients. There are multiple potential explanations for the partial remissions including poor persistence and function of the engineered T cell population due to exposure to multiple rounds of chemotherapy or other toxic agents. In addition, relapse due to loss or modulation of the target antigen on the malignant cell population has been observed. Various strategies are being employed to overcome these limitations of CAR T cell therapy, including dual targeting approaches to additional antigens on the cell surface of the leukemic cells. However, a continued effort into understanding the overall biology of CAR T cell function in a given indication will be critical to realizing the full potential of this novel treatment approach and the development of the next generation of therapies.
NALM6-Luc-mCh-Puro as a Model for B-cell ALL
Robust and reliable preclinical animal models are essential for the effective screening and selection of candidate CAR T therapies for clinical use. We are optimally positioned to provide these models. In 2003, we became the first contract research organization (CRO) to offer in vivo bioluminescence imaging, which is a critical component of studies aimed at determining the efficacy of CAR T cells in hematologic malignancies. We have also made significant efforts to increase our knowledge and experience of preclinical in vivo studies for CAR T cell products to further drug development in this therapeutic spaceâhaving run over 200 studies for more than 24 different clients. Details can be found on our adoptive cell therapy page.
Recently we have expanded our portfolio through the establishment in-house of the NALM6-Luc-mCh-Puro model in NSG mice as a model for ALL. This tumor line was established from the ALL of a 19-year-old male. Similar to the growth kinetics of other well established B-cell malignancy models, such as our Raji-Luc model, the growth of the NALM6-Luc-mCh-Puro tumor cells in 6-7 week old female NSG mice is very robust and reproducible at low cell inocula and demonstrates tumor doubling times in the range of 1 to 1.3 days (Figure 1).
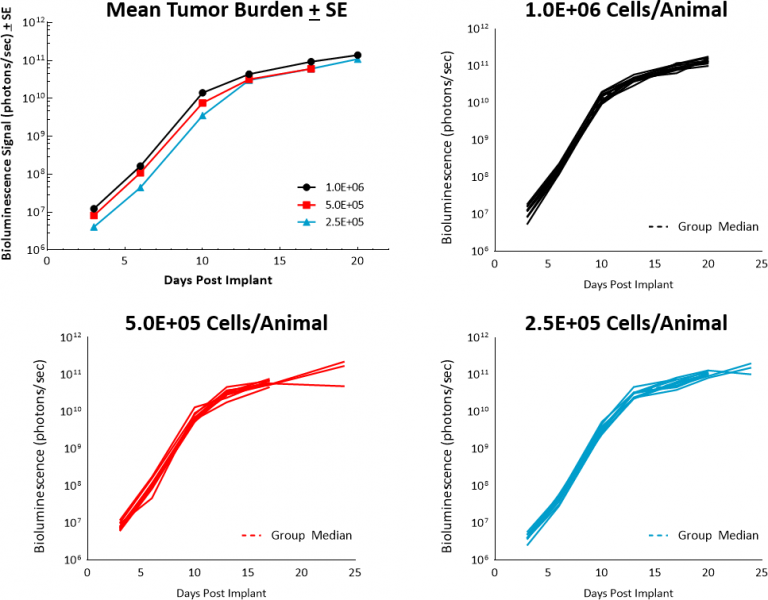
Fig. 1: Growth kinetics for NALM6-Luc-mCh-Puro in NSG mice.
This tumor growth rate results in rapid death in untreated animals, with a median survival time of 24 days (Figure 2). While hind limb paralysis was observed in mice at advanced stages of disease, necropsy findings found evidence of several small nodules in the brain in the majority of euthanized animals. This is of particular interest given the known central nervous system (CNS) disease involvement which is observed in most patients with ALL.
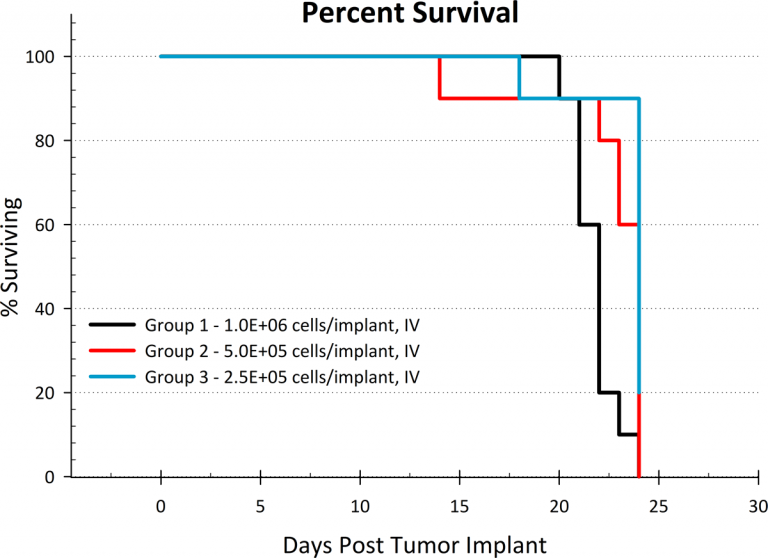
Fig. 2: NALM6-Luc-mCh-Puro median survival plot.
This model provides yet another tool for the preclinical development of therapies directed against B-cell malignancies, particularly in indications aimed at the treatment of ALL. Contact us for your next ALL study or other B-cell malignancy related study.
